Acarology
Smith G, Manzano Marín A, Reyes-Prieto M, Antunes CSR, Ashworth V, Goselle ON, ..., Perotti MA, & Braig H R. 2022
Human follicular mites: Ectoparasites become symbionts.
Molecular Biology and Evolution 39, msac125.
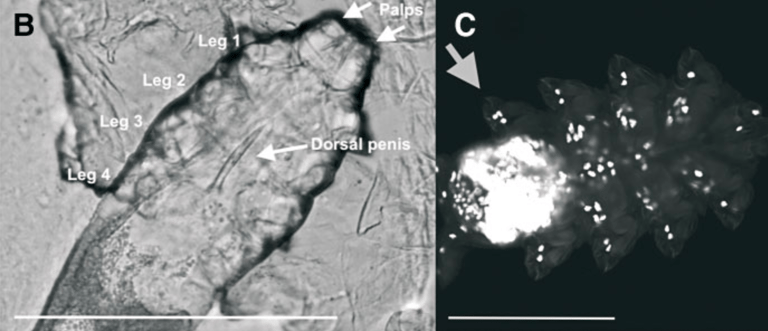
Most humans carry mites in the hair follicles of their skin for their entire lives. Follicular mites are the only metazoans that continuously live on humans. We propose that Demodex folliculorum (Acari) represents a transitional stage from a host-injuring obligate parasite to an obligate symbiont. Here, we describe the profound impact of this transition on the genome and physiology of the mite. Genome sequencing revealed that the permanent host association of D. folliculorum led to an extensive genome reduction through relaxed selection and genetic drift, resulting in the smallest number of protein-coding genes yet identified among panarthropods. Confocal microscopy revealed that this gene loss coincided with an extreme reduction in the number of cells. Single uninucleate muscle cells are sufficient to operate each of the three segments that form each walking leg. While it has been assumed that the reduction of the cell number in parasites starts early in development, we identified a greater total number of cells in the last developmental stage (nymph) than in the terminal adult stage, suggesting that reduction starts at the adult or ultimate stage of development. This is the first evolutionary step in an arthropod species adopting a reductive, parasitic, or endosymbiotic lifestyle. Somatic nuclei show under-replication at the diploid stage. Novel eye structures or photoreceptors as well as a unique human host melatonin-guided day/night rhythm are proposed for the first time. The loss of DNA repair genes coupled with extreme endogamy might have set this mite species on an evolutionary dead-end trajectory.





Perotti MA. 2024
A note from the new Editor-in-Chief.
Experimental and Applied Acarology 92, 307-307.
Since January 2024, I am at the wheel of Experimental and Applied Acarology (EAA). I inherited this precious piece from Jan Bruin, now our Emeritus Editor-in-Chief, to whom I am greatly thankful. Jan not only accompanied EAA growth and success since its launch; but worked hard during the past years with the highest academic integrity, maintaining the Journal’s productivity and quality. My admiration continues to the exceptional acarologists, the founders of EAA, Maus Sabelis, Leo van der Geest and Wim Helle. Thanks to the excellent work of my predecessors and the high quality of contributions of acarologists from around world, a journal with a unique prestige in acarology was generated. And when I say around the world, I mean it. As the world of acarology is reaching new corners of the globe, many more experts develop their careers in the South and in the East. My idea of a new Editorial Board is not to just expand; but enrich and diversify in a way where high calibre scientists bring their knowledge and their own experiences, independently of where they come from. The new Editorial Board forms a living and dynamic body of Editors who interconnect a vast variety of topics.
I am aware of the responsibilities the role of Editor-in-Chief entails, but the challenges are exiting, perhaps a bit tricky at the start. Considering how frequent and fast the natural world is facing changes, scientific writing is also facing changes. Publishing is a learning process not only for the Editors but also for the contributing authors. Challenges can be positive, by generating new ideas, bringing new topics to acarology; and new developments can be easily accommodated in EAA. I am incorporating a number of strong ingredients, types of articles that will surely make EAA more attractive and timely: starting with Opinion Papers, Novel technologies, Best Practice, Curated Datasets, etc. The plan is to stimulate their submission. Not a difficult task, EAA 2022 impact factor (and 5 year IF) raised the journal profile Q1 in the field of Entomology. My goal is to maintain these high standards and continue improving on the Submission to 1st decision (median) rate, which has recently reached to only 14 days!
Farahi S, Shishehbor P, Nemati A, & Perotti MA. 2022
Mesostigmata diversity by manure type: A reference study and new datasets from southwestern Iran.
Experimental and Applied Acarology 86, 517-534.
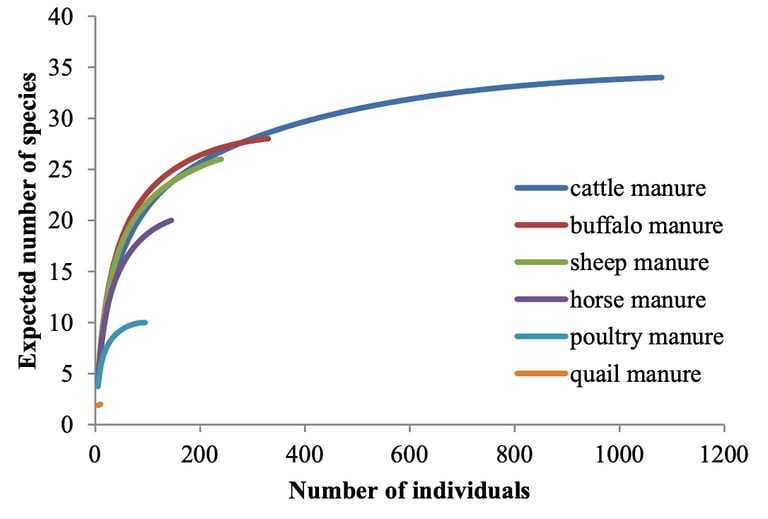
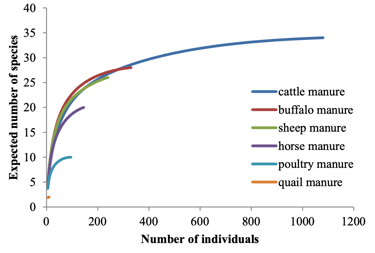
Manure-inhabiting Mesostigmata mites are important biological control agents of pest flies. However, the biodiversity of this mite community is mainly known from Europe and America, and especially from cattle manure. This study examined the diversity and abundance of Mesostigmata mites associated with various types of manure in an (intensive) agricultural region of the Middle East, i.e., the city Ahvaz and its suburbs, in southwest Iran. Mite samples were extracted from manure of cattle, buffalo, sheep, horse, poultry and quail in 30 livestock and poultry farms. In total, 40 species belonging to 24 genera and 16 families were identified. The most diverse families were Laelapidae with eight species, Macrochelidae with seven and Parasitidae with six. Macrocheles muscaedomesticae and Uroobovella marginata were the most widespread species, recorded in 28 and 27 out of 30 collection sites, respectively. Two species, M. sumbaensis and U. marginata, were found in all studied manures. Simpson’s diversity index recorded the highest diversity in buffalo and sheep manure. Real and theoretical species richness (rarefaction curves) were congruent in number of individuals. The presence of seven species of Macrochelidae in the manure confirms that these are important predators of the house fly for the region of Ahvaz and its suburbs. Members of the Parasitidae were highly prevalent, with one species known as a specialized predator of house fly eggs. This work aims to encourage further studies on the diversity of Mesostigmata in these agricultural settings, and further continue assessing the feasibility of these mites as effective biocontrol agents of filth flies in different types of manure and from different corners of the world.
Saloña-Bordas MI, Spooner A, & Perotti MA. 2023
Four hundred and sixty-two mites for the ride: The phoretic companions of a historical Nicrophorus specimen (Coleoptera, Silphidae).
Acarologia 63, 1030-1038.
Burying beetles (Silphidae) and mites (Acari) have a well-established mutualistic interaction, and the phoretic mites will accompany their host even after its death. A voucher specimen of Nicrophorus vespillo (L., 1758) housed in the historical collection at the Oxford University Museum of Natural History (OUMNH) was examined for its 456 plus phoretic mites, which were carefully glued to cardboard slides and preserved together with the beetle carrier. Due to the fragility of the samples, only the Mesostigmata mites were prepared for mounting and further species identification. With the addition of 6 hypopi of Histiostomatidae, a total of 462 mites were identified. Four hypopi were found attached on the Parasitidae dorsal shields and two glued to the cardboard surface. The remaining 456 were composed of 445 specimens of two populations of Poecilochirus carabi Canestrini & Canestrini, 1882 (Parasitidae), and 11 specimens identified as two populations of Macrocheles nataliae Bregetova et Koroleva, 1960. We discuss the interaction and diversity of the numerous mites recorded on a single N. vespillo host. We propose that the presence of different populations of phoretic mites on a single carrier is beneficial to the mites, increasing their chances of outbreeding, adding to their genetic diversity. This study reports a new record of Acari-to-Acari hyperphoresy.
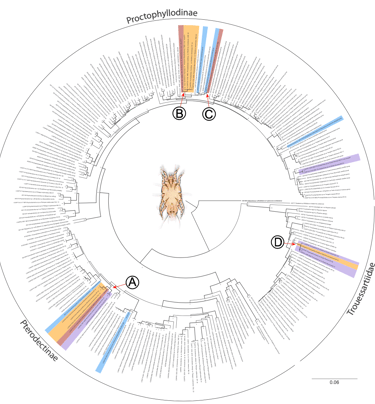
Pedroso LGA, Klimov PB, Mironov SV, OConnor BM, Braig HR, ..., & Hernandes FA, 2023
Horizontal transmission maintains host specificity and codiversification of symbionts in a brood parasitic host.
Communications Biology 6, 1171.
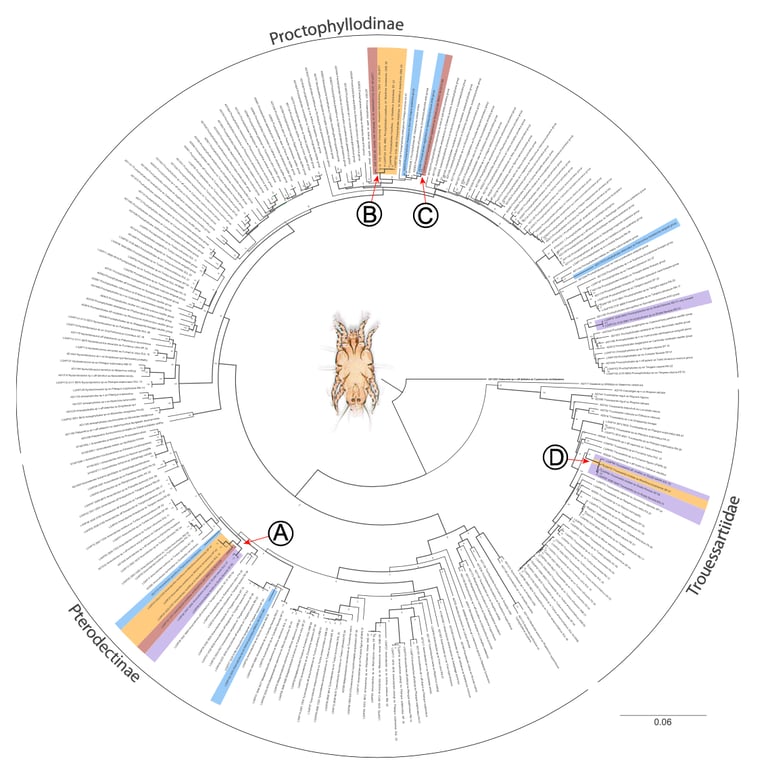
In host-symbiont systems, interspecific transmissions create opportunities for host switches, potentially leading to cophylogenetic incongruence. In contrast, conspecific transmissions often result in high host specificity and congruent cophylogenies. In most bird-feather mite systems, conspecific transmission is considered dominant, while interspecific transmission is supposedly rare. However, while mites typically maintain high host specificity, incongruent cophylogenies are common. To explain this conundrum, we quantify the magnitude of conspecific vs. interspecific transmission in the brood parasitic shiny cowbird (Molothrus bonariensis). M. bonariensis lacks parental care, allowing the assessment of the role of horizontal transmission alone in maintaining host specificity. We found that despite frequent interspecific interactions via foster parental care, mite species dispersing via conspecific horizontal contacts are three times more likely to colonize M. bonariensis than mites transmitted vertically via foster parents. The results highlight the previously underappreciated rate of transmission via horizontal contacts in maintaining host specificity on a microevolutionary scale. On a macroevolutionary scale, however, host switches were estimated to have occurred as frequently as codivergences. This suggests that macroevolutionary patterns resulting from rare events cannot be easily generalized from short-term evolutionary trends.
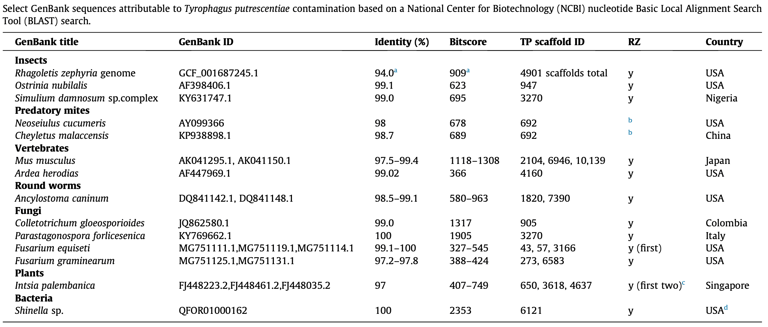
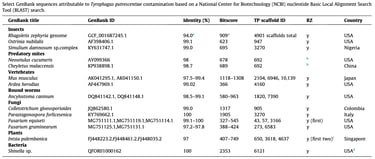
Klimov PB, Cui Y, Hubert J, Erban T, Perotti MA, Braig HR, ..., & Cui Y. 2024
Genomic and metagenomic analyses of the domestic mite Tyrophagus putrescentiae identify it as a widespread environmental contaminant and a host of a basal, mite-specific Wolbachia lineage (supergroup Q).
International Journal of Parasitology, 54, 661-674.
Tyrophagus putrescentiae (mould mite) is a global, microscopic trophic generalist that commonly occurs in various human-created habitats, causing allergies and damaging stored food. Its ubiquity and extraordinary ability to penetrate research samples or cultures through air currents or by active walking through tights spaces (such as treads of screw caps) may lead to sample contamination and introduction of its DNA to research materials in the laboratory. This prompts a thorough investigation into potential sequence contamination in public genomic databases. The trophic success of T. putrescentiae is primarily attributed to the symbiotic bacteria housed in specialized internal mite structures, facilitating adaptation to varied nutritional niches. However, recent work suggests that horizontal transfer of bacterial/fungal genes related to nutritional functionality may also contribute to the mite’s trophic versatility. This aspect requires independent confirmation. Additionally, T. putrescentiae harbors an uncharacterized and genetically divergent bacterium, Wolbachia, displaying blocking and microbiome-modifying effects. The phylogenomic position and supergroup assignment of this bacterium are unknown. Here, we sequenced and assembled the T. putrescentiae genome, analyzed its microbiome, and performed detailed phylogenomic analyses of the mite-specific Wolbachia. We show that T. putrescentiae DNA is a substantial source of contamination of research samples. Its DNA may inadvertently be co-extracted with the DNA of the target organism, eventually leading to sequence contamination in public databases. We identified a diversity of bacterial species associated with T. putrescentiae, including those capable of rapidly developing antibiotic resistance, such as Escherichia coli. Despite the presence of diverse bacterial communities in T. putrescentiae, we did not detect any recent horizontal gene transfers in this mite species and/or in astigmatid (domestic) mites in general. Our phylogenomic analysis of Wolbachia recovered a basal, mite-specific lineage (supergroup Q) represented by two Wolbachia spp. from the mould mite and a gall-inducing plant mite. Fluorescence in situ hybridization confirmed the presence of Wolbachia inside the mould mite. The discovery of an early derivative Wolbachia lineage (supergroup Q) in two phylogenetically unrelated and ecologically dissimilar mites suggests that this endosymbiotic bacterial lineage formed a long-term association with mites. This finding provides a unique insight into the early evolution and host associations of Wolbachia. Further discoveries of Wolbachia diversity in acariform mites are anticipated.
Sumner-Kalkun JC, Baxter I, & Perotti MA. 2020
Microscopic analysis of the microbiota of three commercial Phytoseiidae species (Acari: Mesostigmata).
Experimental and Applied Acarology 81, 389-408.
Pekas A, Palevsky E, Sumner J C, Perotti M A, Nesvorna M, & Hubert J. 2017
Comparison of bacterial microbiota of the predatory mite Neoseiulus cucumeris (Acari: Phytoseiidae) and its factitious prey Tyrophagus putrescentiae (Acari: Acaridae).
Scientific Reports 7, 2.
Miranda RJ, Cleghorn JE, Bermudez SE, & Perotti MA. 2017
Occurrence of the mite Ophionyssus natricis (Acari: Macronyssidae) on captive snakes from Panama.
Acarologia 57, 365-368.
Mašán P, Simpson C, Perotti MA, & Braig HR. 2012
Mites parasitic on Australasian and African spiders found in the pet trade; A redescription of Ljunghia pulleinei Womersley.
PLoS ONE 7, e39019.
Perotti MA. 2001
Prey location and predation rates of predatory mites (Acari: Macrochelidae) on immature stages of pest flies (Diptera: Muscidae).
Systematic and Applied Acarology 6, 27-33.
Perotti MA. 2000
Acaros foréticos (Acari: Pygmephoridae) de la mosca de los cuernos Haematobia irritans (L.). Aspectos ecológicos de la interacción [Phoretic mites of the horn fly, Haematobia irritans (L.). Ecology of the interaction].
Revista de Medicina Veterinaria 80, 449-452.
Perotti MA & Brasesco MJA. 1998
First record of Pediculaster (Acari: Pygmophoridae) for Argentina, collected on the horn fly Haematobia irritans L (Diptera, Muscidae).
Revista de la Sociedad Entomologica Argentina 57, 136.
Perotti MA. 1998
Interacciones entre ácaros (depredadores y foréticos) y dípteros muscoideos (presas y forontes) en hábitats rurales y suburbanos de la pendiente atlántica bonaerense [Predatory and phoretic interactions between mites and flies in the Argentinean pampas (ecology and physiology)]
Universidad Nacional de Mar del Plata, Mar del Plata.
Perotti MA & Brasesco MJA. 1997
Orientación química de Macrocheles muscaedomesticae Scopoli (Acari: Macrochelidae) y percepción a distancia de posturas de Musca domestica (Diptera: Muscidae) y Calliphora vicina (Diptera: Calliphoridae) [Chemo-orientation of Macrocheles muscaedomesticae Scopoli (Acari: Macrochelidae) and detection of distant eggs of Musca domestica (Diptera: Muscidae) and Calliphora vicina (Diptera: Calliphoridae)].
Revista de la Sociedad Entomologica Argentina 56, 67-70.
Perotti MA & Brasesco MJA. 1996
Especificidad forética de Macrocheles muscaedomesticae (Acari: Macrochelidae) [Phoretic specificity of Macrocheles muscaedomesticae (Acari: Macrochelidae)].
Ecología Austral 6, 3-8.